Fixed ratio combinations of a GLP-1 receptor agonist and a basal insulin: 7 pearls
Streamlined simplicity, clinical nuance
There are two fixed ratio combinations (FRCs) of a GLP-1 receptor agonist (GLP1-RA) and a basal insulin in the same product on the market in Canada and other countries in the EU, Australia, UAE, Israel, the US, and others. The products are:
SOLIQUA (SULIQUA in the EU), a combination of lixisenatide and insulin glargine U100 (iGlarLixi). Each mL of iGlarLixi contains 100 units of insulin glarine and 33 mcg of lixisenatide.
XULTOPHY, a combination of liraglutide and insulin degludec (iDegLira). Each mL of iDegLira contains 100 units of insulin degludec and 3.6 mg of liraglutide.
The primarily advantage of these products is the convenience of one pen with two medications (i.e. two drugs in one device formulation).
Here are seven clinical pearls to consider when prescribing these products. Please note this is a curated list of ‘interesting facts’ and one should consult the product monograph and consider the entire clinical picture prior to prescribing any medication.
1. Differences in pharmacokinetics for the GLP-1 receptor agonist component
iGlarLixi (SOLIQUA) contains the GLP1-RA lixisenatide, a once-daily shorter-acting GLP1-RA with a peak absorption between 1 and 3.5 hrs and an elimination half-life of 3 hours. The clinical consequences of a shorter-acting GLP1-RA is they primarily lower post-prandial glucose via delayed gastric emptying. This is why iGlarLixi is to be dosed 1 hr prior to the first meal of the day.
Whereas iDegLira (XULTOPHY) contains the GLP1-RA liraglutide, a once-daily long-acting GLP1-RA with a peak absorption of between 8 and 12 hours and an elimination half-life of 23 hours. Long-acting GLP1-RAs generally have stronger effects on fasting glucose excursions. iDegLira can be dosed any time of day irrespective of meals.
2. Differences in cardiovascular outcome data for the GLP-1 receptor agonist component
Liraglutide demonstrated a 13% reduction in the risk of nonfatal MI, nonfatal stroke, or cardiovascular related-death in the LEADER trial.1 On the other hand, lixisenatide demonstrated non-inferiority for major adverse cardiovascular events compared with placebo in the ELIXA trial.2
3. Slow titration ameliorates adverse gastrointestinal effects of the GLP1-RA component
Due to the dose escalation of both FRC products being based on the basal insulin component, the increase in GLP1-RA dose is very slow compared to a typical dose titration schedule when a GLP1-RA is used on its own. The starting dose of the GLP1-RA component is also generally lower. Let’s take a look at some of the numbers.
When lixisenatide (ADLYXINE) is used on it own, the starting dose is 10 mcg sc once-daily x 14 days and then increased to 20 mcg sc once-daily. That is a dose increase of 10 mcg. For iGlarLixi, the dose increase is much smaller as the titration is based on the basal insulin component. If the basal insulin component is increased by 1-unit, then the lixisenatide increases by ~ 0.3 mcg. The starting dose depends if a patient was previously using basal insulin but could range from a dose 5 mcg of lixisenatide (15 units glargine) in a patient who is insulin naive or using less than 30 units of basal insulin. A dose of 10 mcg of lixisenatide (30 units of glargine) in a patient who is switching from a basal insulin between 30 and less than 60 units.
Liraglutide once-daily (VICTOZA) would be typically started at 0.6 mg sc once-daily for 1 week and then increased to 1.2 mg sc once-daily for further glycemic control. An additional dose escalation to 1.8 mg sc once-daily is an option after 1 week at the 1.2 mg dose. Therefore the dose titration increments are 0.6 mg. However, with iDegLira, the starting dose of liraglutide is either 0.36 mg or 0.58 mg per day with ~ 0.04 mg increments for each unit increase.
Essentially, the GLP1-RA component in these FRC products is micro-dosed. Micro-dosing can also be done off-label with GLP1-RA products such as semaglutide.3
4. Compared to basal insulin alone, less weight gain and less hypoglycemia
The addition of a GLP1-RA at the same time of a basal insulin offsets the potential weight gain and hypoglycemic effects of basal insulin alone. This can be seen in the figure below which shows the average change in weight (left panel) and incidence of hypoglycemia (right panel) from 3 different iGlarLixi trials.4
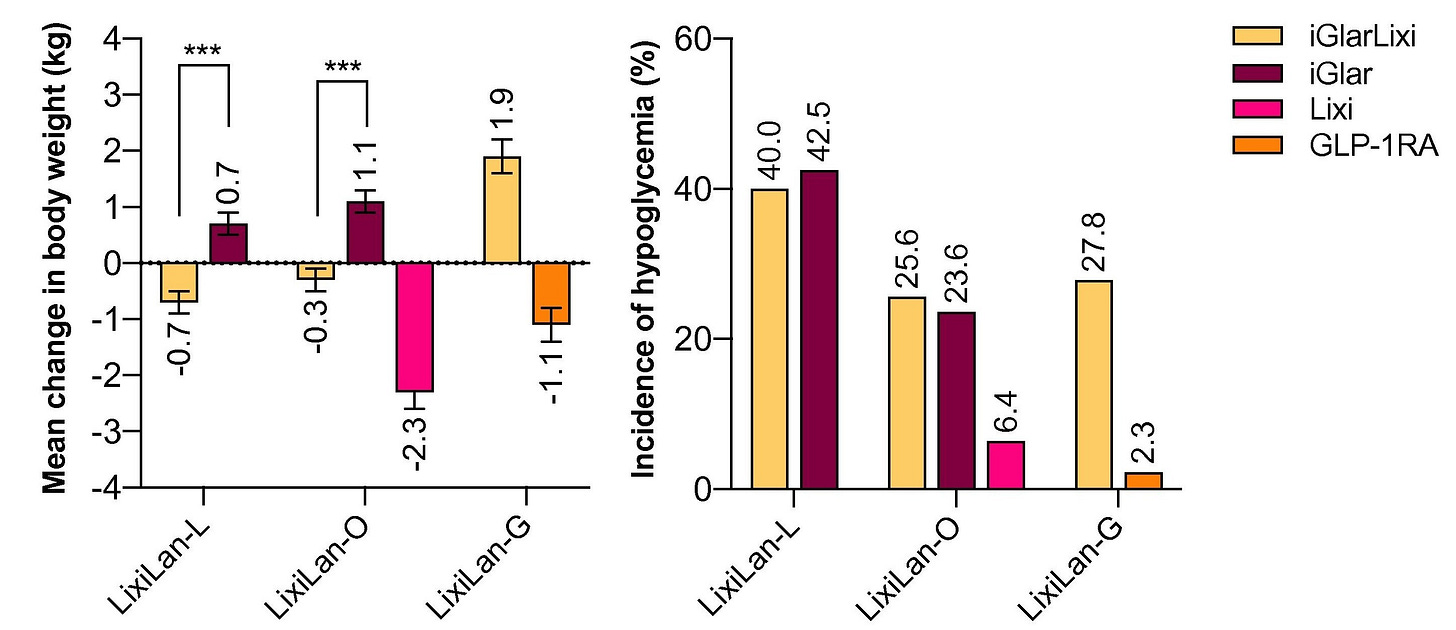
5. Dosing ceilings limit the basal insulin component
Both products are dosed using the basal insulin component and have a limited dosing range.
For iGlarLixi (SOLIQUA), the minimum dose of the basal insulin component (glargine) is 15 units and the maximum dose is 60 units.
For iDegLira (XULTOPHY) the minimum dose of the basal insulin component (degludec) is 10 units and the maximum dose is 50 units.
Therefore if one requires more than the maximum limit of basal insulin and wishes to continue to use the FRC product, then an additional sc injection of the basal insulin component using a separate single entity product is required. Otherwise, switching to separate products is an option (i.e. a basal insulin and a GLP1-RA separately). Note that two injections of the FRC will increase the GLP1-RA component above the intended maximum dose of 20 mcg of lixisenatide for iGlarLixi and 1.8 mg of liraglutide for iDegLira.
6. Few but some contraindications and cautions
The contraindications for these products are the same as GLP-1 receptor agonists including a history of medullary thyroid cancer or MEN2, pregnancy or breastfeeding.
These products are also not recommend in patients with:
gastroparesis - because GLP1-RAs may worsen symptoms of nausea and bloating due to their delayed gastric emptying effect.
gall-bladder disease - cholelithiasis or cholecystitis have been reported in clinical trials of GLP1-RAs.
history of pancreatitis - this is a precaution due to case reports of pancreatitis in patients using lixisenatide or liraglutide during clinical trials.
7. Persons with type 2 diabetes who may benefit from initiating a GLP1-RA / basal insulin FRC product include those with:
Suboptimal HbA1c control on titrated basal insulin, particularly if fasting glucose is under or close to target and post-prandial glucose is elevated
Gastrointestinal intolerance to GLP-1 receptor agonist monotherapy
Concerns about weight gain, hypoglycemia, multiple injections, or regimen complexity
Currently using a GLP1-RA and daily basal insulin within the required dose range (see below regarding limitation of dose ceiling
To be clear, these situations can also be addressed using other strategies but FRCs offer one such option.
That’s it for today’s pharmacotherapy pearl! Stay tuned for upcoming pearls about DPP-4 inhibitors, SGLT-2 inhibitors, and more.
Just in case you missed the previous pharmacotherapy pearl:
Medical Disclaimer: The content in this post is for informational and educational purposes only. It is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the guidance of your physician or other qualified health provider with any questions you may have regarding a medical condition or treatment. Never disregard professional medical advice or delay in seeking it because of something you read here.


