The FLOW Trial
Semaglutide 1mg sc weekly reduces the occurrence of major kidney disease events in patients with type 2 diabetes and chronic kidney disease
FLOW TRIAL. N Engl J Med 2024;391:109-121
Case Vignette: JT, a 67-year-old male with chronic kidney disease, hypertension, and a 15-year history of type 2 diabetes, presents for routine follow-up. His most recent estimated glomerular filtration rate (eGFR) is 47 mL/min/1.73 m² and his urine albumin-to-creatinine ratio (UACR) is 64 mg/mmol (568 mg/g). He is already on maximally tolerated doses of an ARB (irbesartan 300 mg daily), has an A1c of 7.8%, and a BMI of 32 kg/m². He has no prior cardiovascular events, BP is 139/76, and has never smoked. His other medications include atorvastatin 20 mg daily, hydrochlorothiazide 25 mg daily, metformin 1000 mg BID, and insulin glargine U300 42 units qAM. He is interested in weight loss and his A1c target is 6.5%.
In patients such as JT with type 2 diabetes and chronic kidney disease on a maximally tolerated doses of an ARB, further decline in kidney function may be slowed by the addition of an SGLT2 inhibitor or a nsMRA (non-steroidal mineralocorticoid receptor agonist) such as finerenone. The FLOW trial (2024)1 adds compelling evidence for the once weekly subcutaneous GLP-1 receptor agonist semaglutide as a fourth option for renoprotection in patients similar to JT.2
Bottom line result:
Subcutaneous semaglutide 1.0 mg given once weekly significantly reduced the risk of kidney events and cardiovascular death among patients with type 2 diabetes and chronic kidney disease. The number needed to treat was 20 patients to prevent one major kidney event or CV death over 3.4 years.
Take home messages:
The FLOW trial was the first GLP1-RA study designed to evaluate major kidney events as a primary outcome in those with type 2 diabetes and CKD.
The trial was stopped early which could mean the benefits are overstated.
In addition to a reduction in major kidney events (NNT = 20), there was a significant reduction in major adverse cardiovascular events (3-point MACE) with a NNT of 45 and a reduction in all cause mortality (NNT = 39).
Secondary outcomes also showed positive findings with a slower decline in eGFR in the semaglutide group compared to placebo.
SGLT2 inhibitor use was not common at baseline (~16%). However, the use of SGLT2 inhibitors increased during the trial and a prespecified subgroup analysis suggests consistent treatment effects irrespective of SGLT2 inhibitor use at baseline.
Now, let us take a look at the FLOW trial in more detail.
Study design:
Trial type: Placebo-controlled, randomized, parallel-group superiority trial
Randomization: 1:1 treatment allocation stratified by SGLT2 inhibitor use at baseline. Allocation concealed via interactive web response system.
Blinding: Double-blinded. Active treatment and placebo were visually identical.
Population: Adults with type 2 diabetes (glycated hemoglobin level, ≤10%) and chronic kidney disease and receiving a stable maximal tolerated dose of an angiotensin-converting–enzyme (ACE) inhibitor or angiotensin-II-receptor blocker (ARB).
Setting: 387 clinical sites across 28 countries.
Treatment strategies: Weekly subcutaneous semaglutide 1.0 mg versus weekly sc placebo.
Primary endpoint: Composite of major kidney disease events defined as onset of kidney failure, a sustained 50% or greater reduction in eGFR from baseline, death from kidney-related causes, or death from cardiovascular related causes.
Analysis methods: Time to first occurrence of primary composite endpoint.
Summary method: Hazard ratio
Funding: Novo Nordisk
Trial flow:
Very few patients dropped out (<3%); however, about 1 in 4 patients did not complete the treatment as assigned, primarily due to adverse events (12.6% [semaglutide] vs. 11.3% [placebo]).
Who was studied:
All patients had type 2 diabetes and chronic kidney disease (CKD).
CKD was defined by either:
an eGFR of 50 to 75 mL/min/1.73 m2 and a urinary ACR of >33.9 and <565 mg/mmol, OR
an eGFR of 25 to <50 mL/min/1.73 m2 and a urinary ACR of >11.3 and <565 mg/mmol.
Average age ~ 67 years | ~ 30% women | Average HbA1c ~ 7.8% | Average eGFR ~ 47 mL/min/1.73m2 | median ACR ~ 64 mg/mmol.
What were the treatment conditions:
Patients had an 8 week dose escalation period to reach the treatment maintenance dose of semaglutide (or placebo) 1mg sc weekly. The titration was semaglutide (or placebo) 0.25 mg sc weekly for 4 weeks, then semaglutide (or placebo) 0.5 mg sc weekly for 4 weeks prior to increasing to the 1mg weekly dose. Extensions to the dose escalation interval, treatment pauses, or dose reductions were allowed due to intolerance.
The injection was able to administered any time of day without regard to food. The same day of the week was to be used, however, the day could be changed as long as two doses were at least 2 days apart.
Semaglutide or placebo was added to standard of care treatments, although GLP-1 receptor agonists were not allowed to be used during the trial.
Main effects:
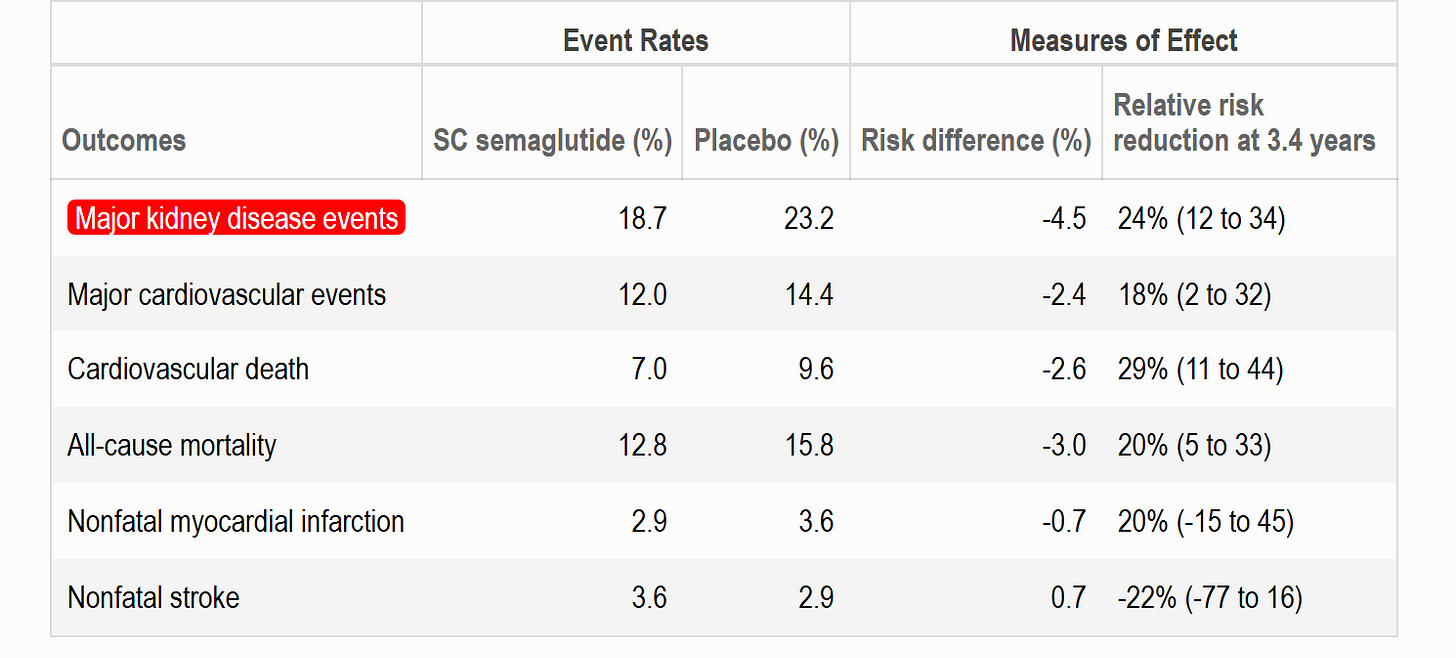
The primary outcome of major kidney disease events was on average 24% less common in the semaglutide group compared to placebo. After 3 years of treatment, about 20 patients needed to be treated with semaglutide 1mg sc weekly to prevent a kidney event in one patient (NNT = 20 [95% CI, 14 to 40]).
The hazard ratios and 95% confidence interval for the primary endpoint and its components are shown in the figure below. Note the significant reduction in a 50% reduction in eGFR and in cardiovascular related death.

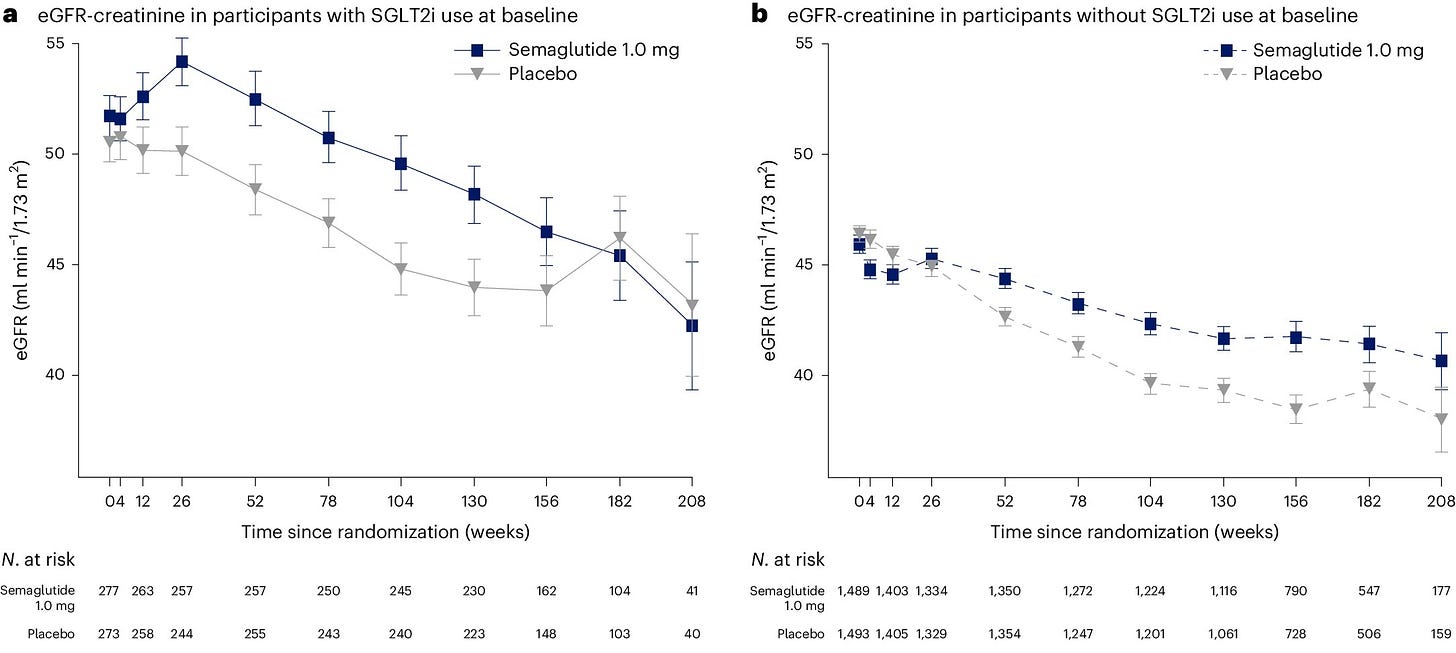
A pre-specified subgroup analysis based on SGLT2 inhibitor use at baseline was published in Nature Medicine.3 Interestingly, at baseline about 16% of trial participants were using an SGLT2 inhibitor. However, during follow-up SGLT2 inhibitors were added on to both the semaglutide and placebo groups as shown below.

The results of this subgroup analysis provide further evidence that the renoprotective effects of semaglutide sc are consistent irrespective of SGLT2 inhibitor use. Recall the HR was 0.76 (95% CI 0.66-0.88) for the primary renal composite endpoint. In patients not using SGLT2 inhibitors at baseline the HR was 0.73 (95% CI 0.63-0.85) and for those using SGLT2 inhibitors at baseline the HR was 1.07 (95% CI 0.69-1.67) with a p-value for the interaction (subgroup difference) of 0.109.
Another interesting finding from the FLOW trial was the secondary endpoint of change in eGFR. There is a steady decline in eGFR in both groups with a slower decline in the semaglutide group.
Lastly, as expected, patients in the semaglutide group experienced greater weight loss (4.1 kg on average), more pronounced HbA1c reduction (average decrease of 0.81%), and decreased systolic blood pressure (average 2.23 mmHg) compared to those in the placebo group.
Safety:
There are no surprises in terms of safety. Serious adverse events were less common in the semaglutide group (figure below) which was driven by fewer serious infestations/infections and fewer serious cardiovascular disorders. Eye disorders were more common is the semaglutide group, although the frequency of diabetic retinopathy adverse events did not differ.
The most common reason for discontinuing semaglutide sc 1 mg weekly was gastrointestinal related adverse events which occurred in 4.5% of patients leading to discontinuation. Treatment discontinuation due to gastrointestinal adverse events was about 4 times more common in the semaglutide group compared to placebo.
Limitations:
In my view, the fact that the FLOW trial was stopped early is the most substantial limitation. The consequences of early termination are potential overestimation of the treatment effect and less long term efficacy and safety data being collected. The magnitude of bias is larger for trials with a smaller number of events, particularly below 500.4 Note that over 700 events were observed in the FLOW trial at truncation.
There were also a few subgroups of interest that are clinically important but not as well represented as some would like. For instance, the small proportion of patients on a SGLT2 inhibitor use at baseline (~16%) may be seen as a limitation; however, as mentioned above SGLT2 inhibitor use increased during the trial and a formal prespecified subgroup analysis did not find an differences in treatment effect by SGLT2 inhibitor use. Also, the percentage of patients with eGFR below 30 mL/min/1.83m2 was low at 11.3% which may limit the generalizability of the study findings.
In context:
As mentioned, the FLOW trial now provides evidence for a 4th treatment option when considering renal protection in patients with type 2 diabetes and CKD. ACE inhibitors and ARBs have been available as a renoprotective treatment strategy for some time with evidence from multiple clinical trials (REIN [1997], IDNT [2001], RENAAL [2001], IRMA-2 [2001], BENEDICT [2004]) supporting their use in patients with type 2 diabetes. More recently, clinical practice guidelines started recommending SGLT2 inhibitors due to their renoprotection effects as demonstrated in the CREDENCE (2019), DAPA-CKD (2022), and EMPA-KIDNEY (2023) trials. Finerenone, a non-steroidal mineralocorticoid receptor antagonist (nsMRA), is another recent addition to the treatment options for renoprotection with evidence from the FIDELITY trial (2024).
All four approaches will slow the downward eGFR trajectory in patients with CKD and type 2 diabetes; however the pattern may be slightly different. For example, an initial dip or drop in eGFR of about 3 to 5 mL/min/1.73m2 within the first month followed by a slower rate (almost flattening) of decline is observed with SGLT2 inhibitors. An small early dip (~2 mL/min1.73m2) is seen with finerenone followed by a continued decline in the slope of eGFR. ACE inhibitors or ARBs generally cause a slow decline in eGFR slope without an initial dip or a small dip. Based on the flow trial, semaglutide has a relatively gentle and steady effect on slowing the eGFR decline without an initial dip. Placebo shows the steepest eGFR decline versus any of the 4 options! The clinical significance of these pattern differences are unknown.
In what order should these drugs be introduced? The 2025 Diabetes Canada Clinical Practice Guideline update on Chronic Kidney Disease in Diabetes2 provides some insight to this question. Maximal label or tolerated doses of ACE inhibitors or ARBs were used as background therapy for most of the SGLT2 inhibitor, finerenone, and semaglutide trials demonstrating renal and cardiovascular protection in patients with type 2 diabetes and CKD. Therefore ACE inhibitors or ARBs are to be started first and then either an SGLT2 inhibitor, finerenone, or semaglutide added. Which agent to add next is a somewhat evidence free zone at the moment. Based on patient preference and clinical priorities, there are several considerations for which agent to add after an ACE inhibitor or ARB.
First, the magnitude of hyperglycemia above target A1c and desirability for weight loss will point to a GLP1-RA (i.e. semaglutide sc) as the preferred agent, followed by an SGLT2i, and then a nsMRA (i.e. finerenone). Finerenone has no substantial effect on A1C or weight. Remember that the glucose lowering effect of SGLT2 inhibitors will diminish as the eGFR declines; however, the renal (and cardio) protective effect remains.
Second, if avoidance of hyperkalemia is a priority then the combination of either an ACE inhibitor or ARB with finerenone should be used with caution.
Third if avoidance of genital infections is a priority then SGLT2 inhibitors should be used with caution.
Fourth, a patient’s willingness to use a product requiring a subcutaneous injection may come into play.
More evidence about combining these renal protective agents was recently published CONFIDENCE trial5 which was published June 5th, 2025 in the NEJM. Briefly, patients with type 2 diabetes and chronic kidney disease on maximally tolerated doses of ACE inhibitors or ARBs were randomized in a 1:1:1 manner to finerenone 10 or 20 mg po daily, empagliflozin 10mg po daily, or a combination of finerenone plus empagliflozin. Matching placebos were used for the monotherapy groups. The primary outcome was a surrogate based endpoint of log-transformed change in urinary ACR from baseline to 6 months post-baseline. The combination group had a greater reduction in urinary ACR compared to either finerenone monotherapy (29% relative risk reduction, 95% CI 18-39) or empagliflozin monotherapy (32% relative risk reduction, 95% CI 21-41).
Has your answered changed for what you would recommend to JT?
Thanks for reading!
Peace and kindness,
JM